Introduction:
Clinical scoring systems, such as the Hemophilia Joint Health Score (HJHS), and imaging modalities (radiographs and magnetic resonance) are important instruments for the evaluation of hemophilic joint health. However, they are semi-quantitative scales, exhibit ceiling effects, and may not capture subtle dynamic changes of intra-articular structures. We developed the Joint tissue Activity and Damage Exam (JADE) protocol to quantitatively evaluate the extent of hemophilic arthropathy at the tissue level, individualize management, and objectively assess the efficacy of novel therapies. JADE is an economical, convenient, and radiation free point-of-care musculoskeletal ultrasound protocol. JADE uses quantitative numerical measurements (1/10th of millimeter) for osteochondral alterations, cartilage thickness and soft tissue expansion in hemophilic joints and is validated per international OMERACT guidelines for pathological tissue recognition with high intra/inter-rater and inter-operator reliability. Furthermore, we previously reported JADE association with clinical and functional joint assessments at baseline for patients with hemophilia.
Herein, we examined JADE protocol ability to capture tissue changes over time and their associations with HJHS, a clinical joint assessment. We assumed the regression lines for joint HJHS on each JADE measurement at each time point would lie parallel to each other. Verifying this assumption would enhance JADE protocol validity and provide confidence that it could be used to monitor hemophilic arthropathy progression by quantifying specific tissue abnormalities.
Methodology:
We recruited adult patients (≥ 18 years) with congenital hemophilia and arthropathy in a prospective study performed at 3 sites in North America. We assessed joint HJHS and JADE parameters for each patient (n=44; 264 joints [bilateral elbows, ankles, and knees]) at study entry (baseline), at ~12-18 months (midpoint), and ~24-36 months (final). JADE measurements included osteochondral alterations, cartilage thickness, and soft tissue expansion at sentinel positions for each joint. The association between joint HJHS and each JADE variable was examined by fitting random intercept models with outcomes transformed to ensure normal residuals. We examined the assumption of parallel regression lines for the three time points by applying a test for homogeneity of slopes. If no difference between slopes was found, then we applied an analysis of covariance to test whether the lines for each joint were coincident.
Results:
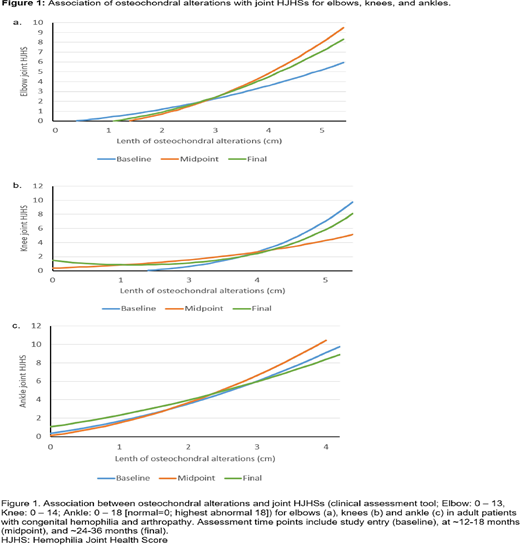
Joint HJHSs deteriorated measurably during the study period, reflecting clinically worsening arthropathy over time. Importantly, clinical assessment of deterioration for the elbows, knees, and ankles was associated with JADE measurements that changed in the expected direction, namely increased length of osteochondral alterations, decreased cartilage thickness, and increased soft tissue expansion. As an example, figure 1 shows the association of deteriorating HJHS and osteochondral alterations for elbows, knees, and ankles at baseline, midpoint, and final visits. In each graph, the regression lines were similar in shape. Lines were parallel for the elbow (midpoint vs baseline: p = 0.185; final vs baseline: p = 0.398), and the ankle (midpoint vs baseline: p = 0.225; final vs baseline: p = 0.293). But, the assumption of parallel lines was rejected for the knee (midpoint vs baseline: p = 0.047; final vs baseline: p = 0.024).
Conclusions:
This study demonstrates a tight association between JADE direct ultrasonographic measurements and clinical deterioration (HJHSs) during several assessments over 3 years. Importantly, the associations are consistent across time, despite clinical joint health examinations and ultrasound measurements being performed at three different centers by multiple providers, with long intervals between assessments. Altogether, these findings constitute a major milestone for the clinical relevance validity of the JADE protocol as a precise instrument for tissue-specific alterations measurements over time. These findings support the use of the JADE protocol to monitor the progression of hemophilic arthropathy longitudinally, permit prompt identification of potentially reversible osteochondral and/or soft tissue changes, and study early interventions that could curb arthropathic progression.
Kruse-Jarres:F. Hoffmann-La Roche Ltd: Speakers Bureau; Biomarin, Chugai Pharmaceutical Co., CSL Behring, CRISPR Therapeutics, Genentech, Inc.: Honoraria; CSL Behring, Genentech, Inc., Spark: Research Funding; Biomarin, Chugai Pharmaceutical Co., CSL Behring, CRISPR Therapeutics, Genentech, Inc.: Consultancy. Steiner:Uniqure: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi/Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Quon:Bayer: Honoraria; Orthopaedic Institute for Children: Current Employment; Biomarin: Honoraria, Speakers Bureau; Novo Nordisk: Honoraria, Speakers Bureau; Bioverativ/Sanofi: Honoraria, Speakers Bureau; Genentech, Inc./F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau; Octapharma: Honoraria; Shire/Takeda: Speakers Bureau. von Drygalski:Biomarin: Honoraria; Hematherix LLC: Membership on an entity's Board of Directors or advisory committees, Other: co-founder; Uniqure: Honoraria; Bioverativ/Sanofi: Honoraria, Research Funding; Pfizer: Research Funding; Novo-Nordisk: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.